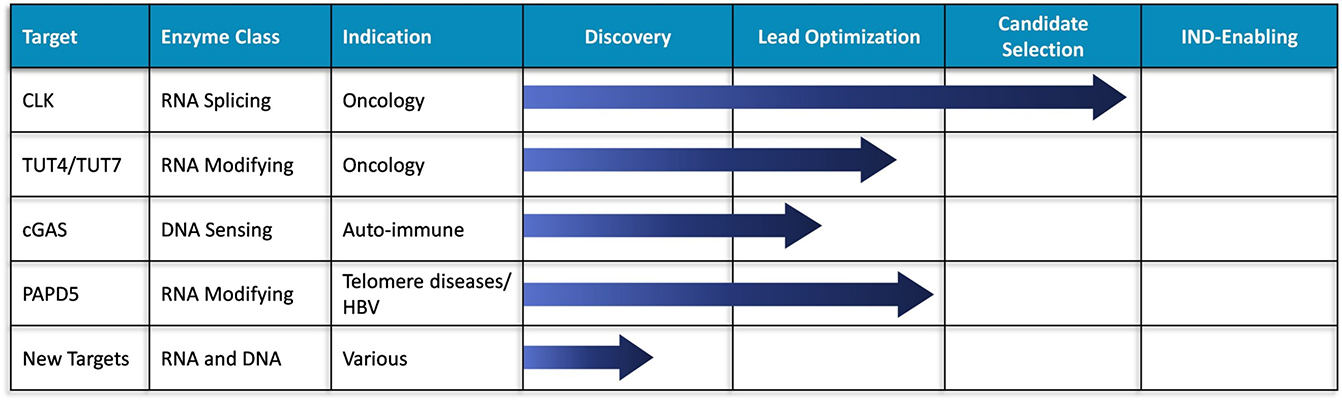
Exciting Pipeline of Novel Programs
Our scientific expertise and proprietary know-how have been successfully applied to a diverse set of RNA and DNA processing and sensing enzymes to rapidly generate and advance a series of exciting programs with exciting animal data that are moving rapidly toward the clinic.
CLK Program
Splicing is an important process of converting pre-mRNA to mature mRNA for translation into proteins. Over 95% of RNA is alternatively spliced to create a diversity of protein products from a given gene.
Aberrant RNA splicing occurs in a number of cancers and is believed to be a driver of oncogenesis. There is growing understanding of specific proteins, including enzymes, and other factors involved in aberrant splicing and their role in diseases.
CLK (CDC-like kinase) is a family of four enzymes (CLK 1 – 4) that acts on proteins in the spliceosome complex. CLK over-expression has been functionally tied to aberrant RNA splicing in a variety of cancers including solid tumors (lung, breast, colorectal, pancreatic) and heme malignancies (AML, CML, MDS).
CLK inhibitors have demonstrated the ability to inhibit cancer cell growth and induce apoptosis through splicing alternations in genes involved in growth and cell survival.
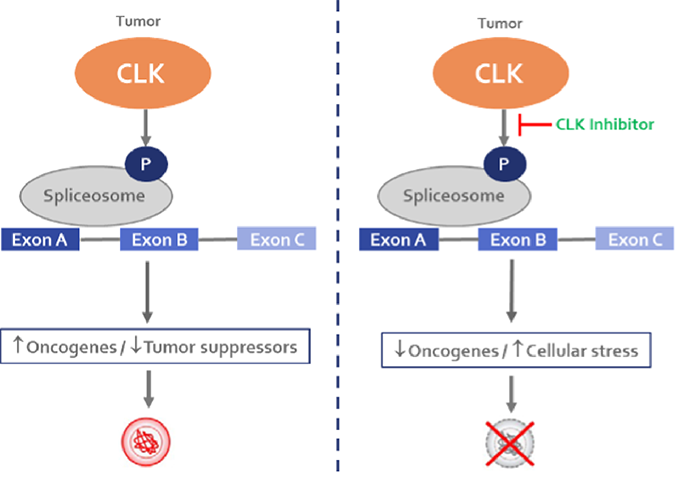
Redona is developing a potential best-in-class small molecule CLK inhibitor that may provide benefit across a range of solid tumors with high unmet need. The company also has generated proprietary data that will enable tailored patient enrichment and selection strategies in clinical studies.
TUT4/TUT7 Program
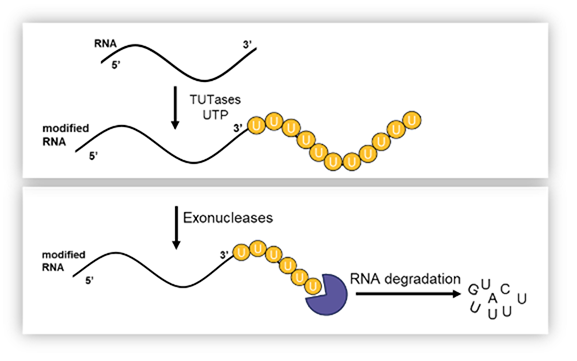
RNA homeostasis is a highly regulated process governed by a multitude of cellular pathways. The rates of RNA synthesis and degradation are among the most important factors for gene regulation. Poly-uridylation of the 3’-end of mRNA is well established as one of the key mechanisms to mark RNA for degradation by various exonucleases.
RNA uridylation is performed by various terminal uridyl transferase (TUT) enzymes. TUT4 and TUT7 function redundantly and are found to be upregulated in certain cancers. Genetic ablation as well as pharmacological inhibition of TUT4/7 have demonstrated compelling efficacy in both cell and animal models of cancer.
In addition, a strong association between TUT4/7 inhibitor sensitivity and a specific genetic deletion found in certain cancers has been identified that will aid in developing a robust patient selection strategy for clinical studies.
Redona has developed a series of potent and selective dual inhibitors of both TUT4 and TUT7 (TUT4/7) that have demonstrated compelling efficacy in models of solid cancers.
CGAS Program
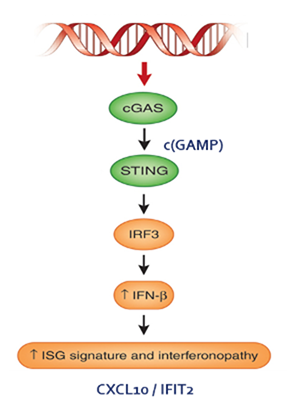
cGAS (cyclic GMP-AMP synthase) is a sensor of cytosolic DNA generated by infections, tissue damage, cancer, and auto-immune diseases.
Activation of the cGAS signaling pathway leads to the expression of pro-inflammatory genes. The broad activation of these pathways by cGAS provides potential therapeutic opportunities for intervention in a wide range of indications including autoimmune diseases and cancer.
Redona has developed a series inhibitors that are potent and selective against cGAS, that cross-react between human and mouse targets, possess excellent cell penetration and oral PK properties, and demonstrated convincing cGAS inhibition in animal models.
PAPD5/PAPD7 Program
PAPD5 and PAPD7 are members of a family of poly(A)polymerases that append multiple adenyl groups onto the ends of RNA to stabilize the nucleic acid. PAPD5/7 are cellular host factors required for HBV RNA stabilization and PAPD5/7 inhibitors are considered an important component of HBV combination therapy needed to produce a functional cure. PAPD5 poly-adenylates and destabilizes TERC (telomerase RNA component), an important factor required for telomere maintenance, and PAPD5 inhibitors may have benefit for rare genetic diseases with telomere dysfunction such as dyskeratosis congenita that leads to bone marrow failure, liver cirrhosis and pulmonary fibrosis.
Redona has developed an advanced series of highly potent and selective PAPD5/7 inhibitors that contain a distinctly unique chemical scaffold relative to all known competitor molecules. Our lead compounds have excellent oral bioavailability and have demonstrated activity in animal models of HBV infection.
Pipeline & Partnering
We welcome the opportunity to:
- Partner our compounds in non-oncology settings
- Explore new technologies and therapeutics for RNA applications